Talk
ZARM Talk: Treasured Water Electrolysis for the Future
Water electrolysis was discovered about 200 years ago. On November 20, Hisayoshi Matsushima from the Hokkaido Univeristy in Japan explains how electrolysis will be applied for a future energy system for space and nuclear fusion.
ZARM Talk by Hisayoshi Matsushima
from the Hokkaido Univeristy
- Date: 20 November,
- Time: 14:00
- Location: ZARM, room 1730
In 1800, Anthony Carlisle and William Nicholson performed water electrolysis first. Nearly 200 years later, the electrolysis is applied for advance energy fields.
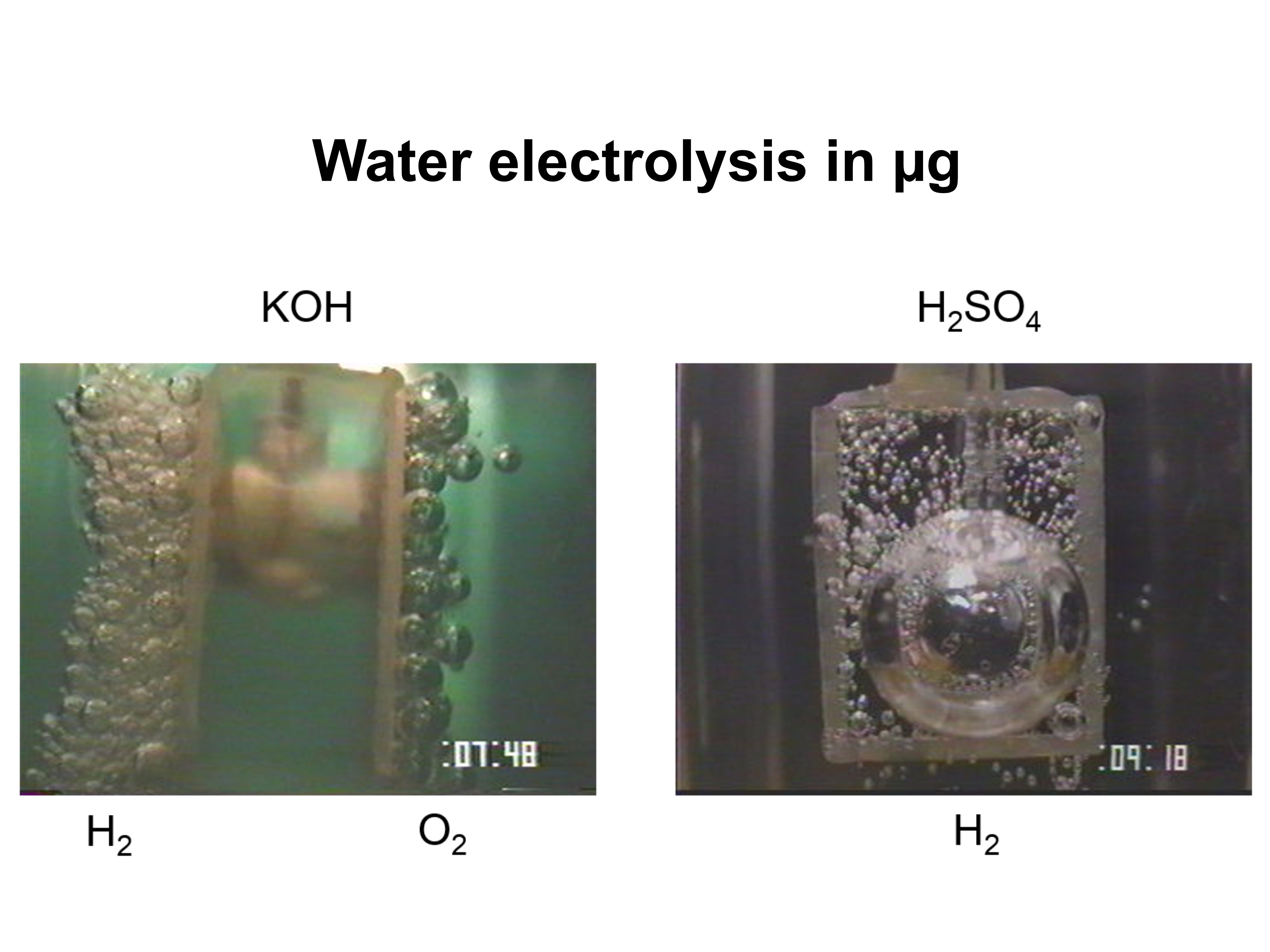
The use of hydrogen energy system is considered for lunar bases. The electric energy by solar power is converted/stored as hydrogen gas via water electrolysis during daytime, then regenerated into electricity by fuel cells at night. Under normal gravity, buoyancy causes the generated gas to separate from the electrode surface naturally. However, under microgravity, bubbles cannot detach from the electrode surface. Consequently, electrolysis in space has drawbacks of high-power consumption. Therefore, it is necessary to in-situly observe and evaluate the characteristics of the bubbles generated during electrolysis.
On our Earth, water electrolysis gets attention not only for hydrogen gas production but also for a new application, namely hydrogen isotope separation. Hydrogen exists as isotopes; protium, deuterium, and tritium. During electrolysis, light substances form gas easily. This property is applied in the recycling of fuels (deuterium and tritium) and in cooling water treatment for fusion reactors.
In this lecture, I will introduce my previous research on water electrolysis using a falling-film tower. I will also present unique research currently undertaking: deuterium separation via water electrolysis and the formation of electrolytic nanobubbles using high-speed atomic force microscopy.