Heat Transfer and Convective Flows
Claudia Zimmermann
The computational description of evaporation processes requires the calculation of the heat and mass transfer exchange rate over the liquid/gas interface. The most influential parameter for the exchange rates is the relative humidity of the gas phase. The highest relative humidity gradient occurs at temperature levels slightly below the boiling point. As a result, these temperatures have to be considered when computing the dispersed phase evaporation driven by temperature increase.
The dimensionless numbers characterizing the heat and mass exchange process are the Nusselt number Nu and the Sherwood number Sh. Several approaches based on the correlation model of Ranz and Marschall, have been developed in order to model these parameters. The evaporation rate model of Abramzon and Sirignano additionally considers the latent heat flux of the evaporated liquid leaving the droplet. The correct definition of the gas phase humidity requires the mass ratio of the liquid's vapor in the gas phase Y, which is influenced by convective, conductive, turbulent and thermal diffusive effects, to be computed from an appropriate transport equation in addition to the one governing the temperature field T.
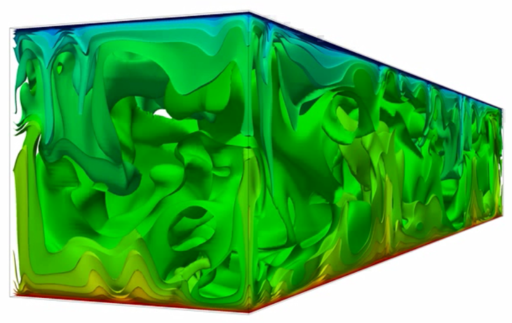
For all subcritical and supercritical thermo fluid-dynamic processes the second law of thermodynamics, non-negative production of entropy, has to be satisfied. Models describing thermo-fluid dynamic processes, which comply with the second law of thermodynamics, are called thermodynamic consistent. When describing macroscopic thermodynamic processes with stochastic approaches of molecular motion, the thermodynamic consistence must be proven by the second-law analysis (SLA). The resulting consistent entropy production, the comparison between macroscopic physical values and the molecular diffusion modeling are some of the main advantages of this approach.


 "
"